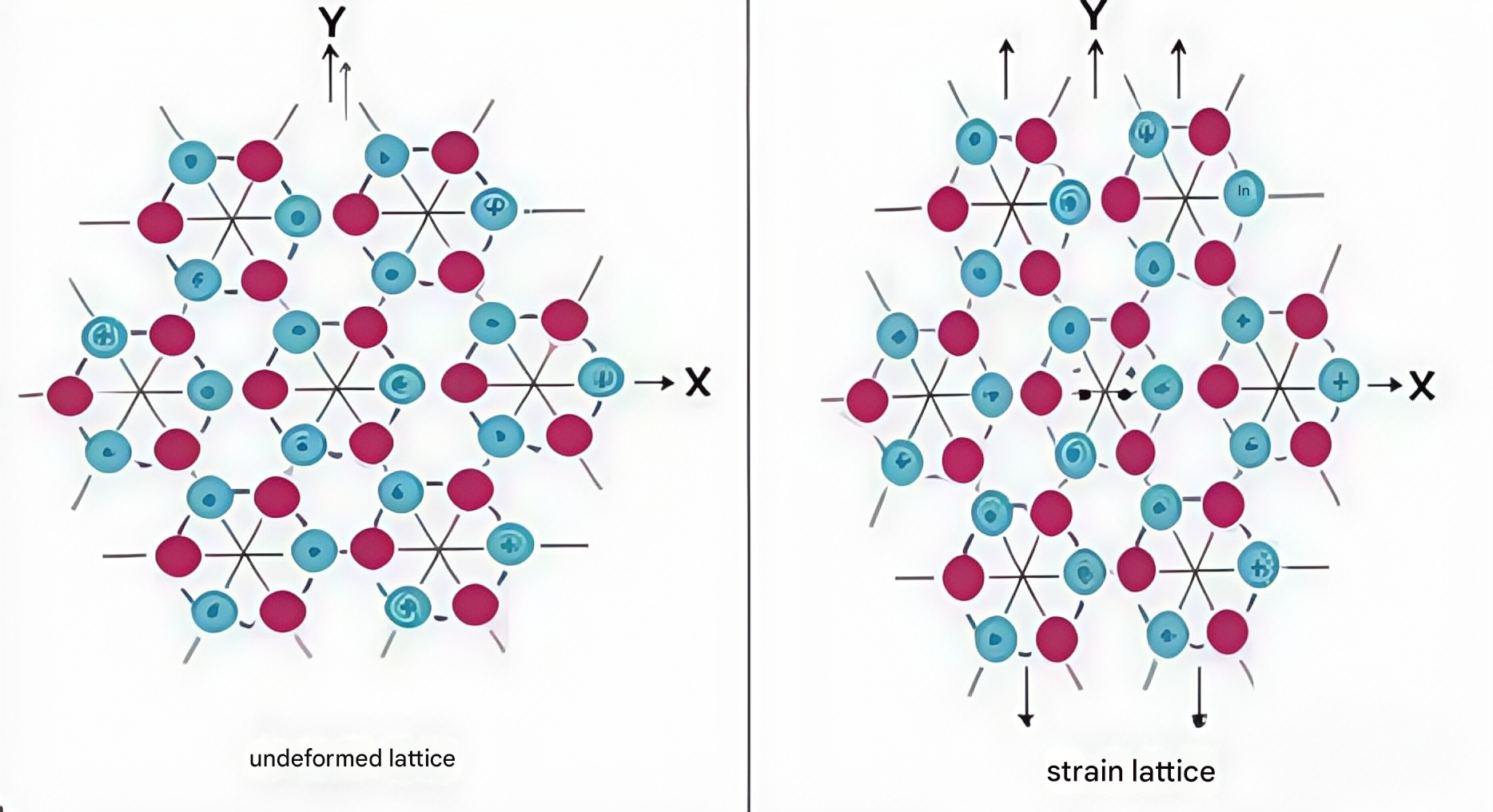
1. Thermal Stress During Cooling (Primary Cause)
Fused quartz generates stress under non-uniform temperature conditions. At any given temperature, the atomic structure of fused quartz reaches a relatively "optimal" spatial configuration. As temperature changes, atomic spacing shifts accordingly—a phenomenon commonly referred to as thermal expansion. When fused quartz is unevenly heated or cooled, non-uniform expansion occurs.
Thermal stress typically arises when hotter regions attempt to expand but are constrained by surrounding cooler zones. This creates compressive stress, which usually does not cause damage. If the temperature is sufficiently high to soften the glass, the stress can be relieved. However, if the cooling rate is too fast, the viscosity increases rapidly, and the internal atomic structure cannot adjust in time to the decreasing temperature. This results in tensile stress, which is much more likely to cause fractures or failure.
Such stress intensifies as temperature drops, reaching high levels at the end of the cooling process. The temperature at which quartz glass reaches a viscosity above 10^4.6 poise is referred to as the strain point. At this point, the material's viscosity is so high that internal stress becomes effectively locked in and can no longer dissipate.
2. Stress from Phase Transition and Structural Relaxation
Metastable Structural Relaxation:
In the molten state, fused quartz exhibits a highly disordered atomic arrangement. Upon cooling, atoms tend to relax toward a more stable configuration. However, the high viscosity of the glassy state hinders atomic movement, resulting in a metastable internal structure and generating relaxation stress. Over time, this stress may be slowly released, a phenomenon known as glass aging.
Crystallization Tendency:
If fused quartz is held within certain temperature ranges (such as near the crystallization temperature) for extended periods, microcrystallization may occur—for instance, the precipitation of cristobalite microcrystals. The volumetric mismatch between crystalline and amorphous phases creates phase transition stress.
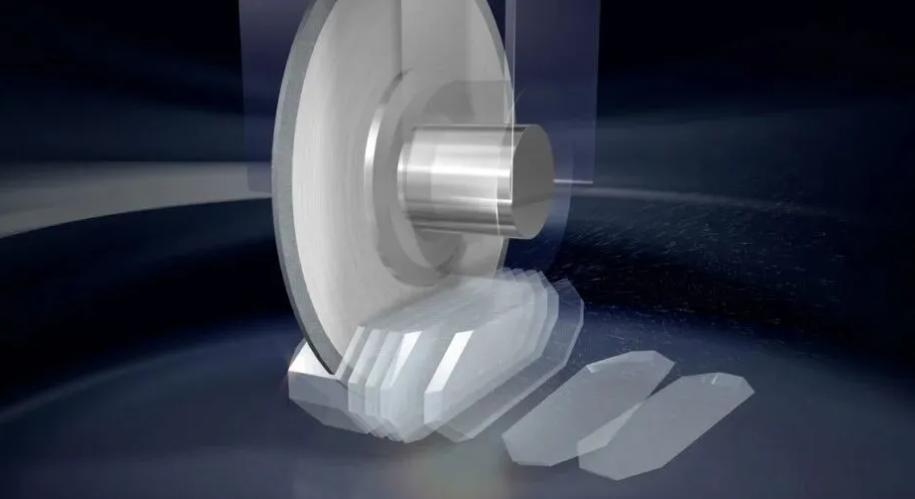
3. Mechanical Load and External Force
1. Stress from Processing:
Mechanical forces applied during cutting, grinding, or polishing can introduce surface lattice distortion and processing stress. For example, during cutting with a grinding wheel, localized heat and mechanical pressure at the edge induce stress concentration. Improper techniques in drilling or slotting can lead to stress concentrations at notches, serving as crack initiation points.
2. Stress from Service Conditions:
When used as a structural material, fused quartz can experience macro-scale stress due to mechanical loads such as pressure or bending. For instance, quartz glassware may develop bending stress when holding heavy contents.
4. Thermal Shock and Rapid Temperature Fluctuation
1. Instantaneous Stress from Rapid Heating/Cooling:
Although fused quartz has a very low thermal expansion coefficient (~0.5×10⁻⁶/°C), rapid temperature changes (e.g., heating from room temperature to high temperatures, or immersion in ice water) can still cause steep local temperature gradients. These gradients result in sudden thermal expansion or contraction, producing instantaneous thermal stress. A common example is laboratory quartzware fracturing due to thermal shock.
2. Cyclic Thermal Fatigue:
When exposed to long-term, repeated temperature fluctuations—such as in furnace linings or high-temperature viewing windows—fused quartz undergoes cyclic expansion and contraction. This leads to fatigue stress accumulation, accelerating aging and the risk of cracking.

5. Chemically Induced Stress
1. Corrosion and Dissolution Stress:
When fused quartz comes into contact with strong alkaline solutions (e.g., NaOH) or high-temperature acidic gases (e.g., HF), surface corrosion and dissolution occur. This disrupts structural uniformity and induces chemical stress. For example, alkali corrosion can lead to surface volume changes or microcrack formation.
2. CVD-Induced Stress:
Chemical Vapor Deposition (CVD) processes that deposit coatings (e.g., SiC) onto fused quartz can introduce interfacial stress due to differences in thermal expansion coefficients or elastic moduli between the two materials. During cooling, this stress can cause delamination or cracking of the coating or substrate.
6. Internal Defects and Impurities
1. Bubbles and Inclusions:
Residual gas bubbles or impurities (e.g., metallic ions or unmelted particles) introduced during melting can serve as stress concentrators. Differences in thermal expansion or elasticity between these inclusions and the glass matrix create localized internal stress. Cracks often initiate at the edges of these imperfections.
2. Microcracks and Structural Flaws:
Impurities or flaws in the raw material or from the melting process may result in internal microcracks. Under mechanical loads or thermal cycling, stress concentration at crack tips can promote crack propagation, reducing material integrity.
Post time: Jul-04-2025
